New Process Chemistry paper in collaboration with Angelini Pharma
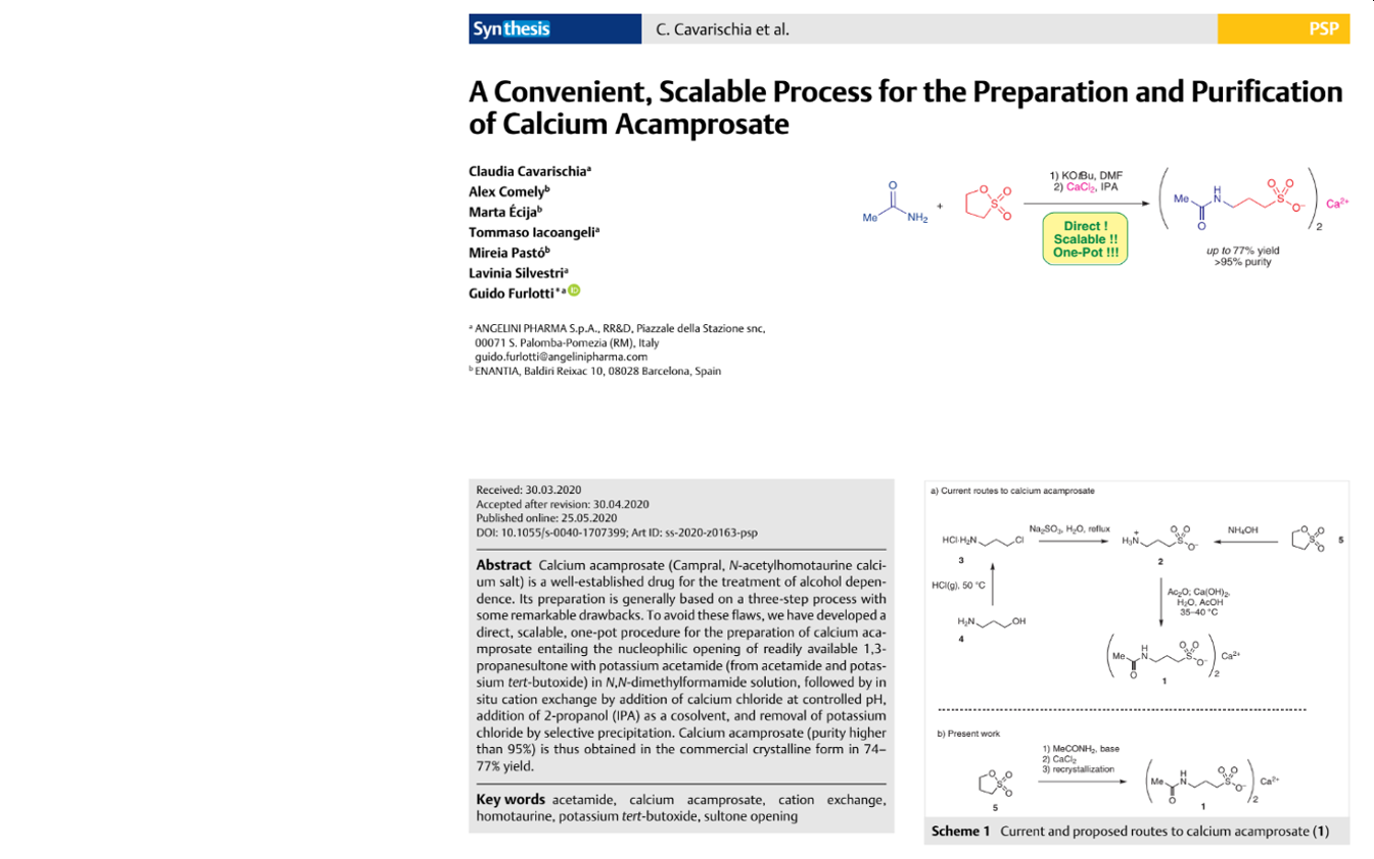
Within this collaboration, a new robust and scalable synthetic route to obtain calcium acamprosate has been developed. The selective precipitation of calcium acamprosate as an easily filterable solid with 74–77% yield was achieved by the one-pot nucleophilic opening of 1,3-propanesultone with potassium acetamide, following by cation exchange with calcium ion at controlled pH.
In comparison with other procedures described in the literature and used previously, the newly developed and optimized methodology gives rise to a remarkably more efficient and direct synthesis of calcium acamprosate, avoiding the use of toxic chemicals and multistep synthesis.
This is a perfect example of how our process chemistry team can support our customers in designing and optimizing new routes of synthesis, leading to procedures that can be easily scaled up and used later at industrial scale. Furthermore, this type of projects can be complemented at Enantia by performing the carry-over of the impurities, and the characterization thereof.
You can read the full paper here.


